Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
What
does the constant bombardment of gas molecules against the inside walls of a container
produce? a. | temperature | c. | pressure | b. | density | d. | diffusion | | | | |
|
|
|
2.
|
Standard temperature is exactly a. | 100ºC. | c. | 0ºC. | b. | 273ºC. | d. | 0
K. | | | | |
|
|
|
3.
|
Standard pressure is exactly a. | 1 atm. | c. | 101.325 atm. | b. | 760
atm. | d. | 101
atm. | | | | |
|
|
|
4.
|
The
volume of a gas is 400.0 mL when the pressure is 1.00 atm. At the same temperature, what is the
pressure at which the volume of the gas is 2.0 L? a. | 0.5 atm | c. | 0.20 atm | b. | 5.0
atm | d. | 800
atm | | | | |
|
|
|
5.
|
A
sample of oxygen occupies 560. mL when the pressure is 800.00 mm Hg. At constant temperature, what
volume does the gas occupy when the pressure decreases to 700.0 mm Hg? a. | 80.0
mL | c. | 600.
mL | b. | 490.
mL | d. | 640.
mL | | | | |
|
|
|
6.
|
The
volume of a gas is 5.0 L when the temperature is 5.0ºC. If the temperature is increased to
10.0ºC without changing the pressure, what is the new volume? a. | 2.5
L | c. | 5.1
L | b. | 4.8
L | d. | 10.0
L | | | | |
|
|
|
7.
|
At
7.0ºC, the volume of a gas is 49 mL. At the same pressure, its volume is 74 mL at what
temperature? a. | 3.0ºC | c. | 120ºC | b. | 423ºC | d. | 150ºC | | | | |
|
|
|
8.
|
The
volume of a gas is 93 mL when the temperature is 91ºC. If the temperature is reduced to 0ºC
without changing the pressure, what is the new volume of the gas? a. | 70.
mL | c. | 120
mL | b. | 93
mL | d. | 273
mL | | | | |
|
|
|
9.
|
On a
cold winter morning when the temperature is –13ºC, the air pressure in an automobile tire
is 1.5 atm. If the volume does not change, what is the pressure after the tire has warmed to
15ºC? a. | –1.5
atm | c. | 3.0
atm | b. | 1.7
atm | d. | 19.5
atm | | | | |
|
|
|
10.
|
The
pressure of a sample of gas at 10.0ºC increases from 700. mm Hg to 900. mm Hg. What is the new
temperature? a. | 0ºC | c. | 39.0ºC | b. | 364ºC | d. | 90.9ºC | | | | |
|
|
|
11.
|
The
volume of a sample of oxygen is 300.0 mL when the pressure is 1.00 atm and the temperature is
27.0ºC. At what temperature is the volume 1.00 L and the pressure 0.500 atm? a. | 22.0ºC | c. | 0.50
K | b. | 45.0ºC | d. | 227ºC | | | | |
|
|
|
12.
|
The
volume of a gas collected when the temperature is 11.0ºC and the pressure is 710 mm Hg measures
14.8 mL. What is the calculated volume of the gas at 20.0ºC and 740 mm Hg? a. | 7.8
mL | c. | 14.6
mL | b. | 13.7
mL | d. | 15
mL | | | | |
|
|
|
13.
|
In
the reaction represented by the equation N2(g) + 2O2(g)
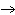 2NO2(g), what is the volume ratio of N2 to
NO2? 2NO2(g), what is the volume ratio of N2 to
NO2?
|
|
|
14.
|
The
equation for the production of methane is C + 2H2(g) 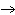 CH4(g). How many liters of hydrogen are needed to produce 20. L of
methane? CH4(g). How many liters of hydrogen are needed to produce 20. L of
methane? a. | 2.0
L | c. | 22.4
L | b. | 20.
L | d. | 40.
L | | | | |
|
|
|
15.
|
What
is the number of moles of H2 produced when 23 g of sodium react with water according to
the equation 2Na(s) + 2H2O(l) 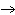 2NaOH(aq) + H2(g)?
2NaOH(aq) + H2(g)? a. | 0.50 mol | c. | 2.0 mol | b. | 1.0
mol | d. | 4.0
mol | | | | |
|
|
|
16.
|
The
principle that under similar pressures and temperatures, equal volumes of gases contain the same
number of molecules is attributed to a. | Boyle. | c. | Avogadro. | b. | Graham. | d. | Dalton. | | | | |
|
|
|
17.
|
The
standard molar volume of a gas at STP is a. | 22.4 L. | c. | g-mol wt/22.4 L. | b. | g/22.4
L. | d. | 1
L. | | | | |
|
|
|
18.
|
A
1.00 L sample of a gas has a mass of 1.92 g at STP. What is the molar mass of the
gas? a. | 1.92
g/mol | c. | 22.4
g/mol | b. | 19.2 g/mol | d. | 43.0 g/mol | | | | |
|
|
|
19.
|
A
1.00 L sample of a gas has a mass of 1.25 g at STP. What is the mass of 1.00 mol of this
gas? a. | 0.855
g | c. | 22.4
g | b. | 1.25
g | d. | 28.0
g | | | | |
|
|
|
20.
|
What
is the value of the gas constant?
|
|
|
21.
|
Calculate the approximate volume of a 0.600 mol sample of gas at 15.0°C and a
pressure of 1.10 atm. a. | 12.9 L | c. | 24.6 L | b. | 22.4
L | d. | 139
L | | | | |
|
|
|
22.
|
Calculate the approximate temperature of a 0.50 mol sample of gas at 750 mm Hg and a
volume of 12 L. a. | –7ºC | c. | 15ºC | b. | 11ºC | d. | 288ºC | | | | |
|
|
|
23.
|
What
is the pressure exerted by 1.2 mol of a gas with a temperature of 20.ºC and a volume of 9.5
L? a. | 0.030
atm | c. | 3.0
atm | b. | 1.0
atm | d. | 30.
atm | | | | |
|
|
|
24.
|
A
sample of gas at 25ºC has a volume of 11 L and exerts a pressure of 660 mm Hg. How many moles of
gas are in the sample? a. | 0.39 mol | c. | 9.3 mol | b. | 3.9
mol | d. | 87
mol | | | | |
|
|
|
25.
|
A gas
sample with a mass of 12.8 g exerts a pressure of 1.2 atm at 15ºC and a volume of 3.94 L. What
is the molar mass of the gas? a. | 19 g/mol | c. | 64 g/mol | b. | 32
g/mol | d. | 128
g/mol | | | | |
|
|
|
26.
|
A gas
is confined in a steel tank with a volume of 6.982 L. At 20.20°C, the gas exerts a pressure of
8.532 atm. After heating the tank, the pressure of the gas increases to 10.406 atm. What is the
temperature of the heated gas? a. | –32.60°C | c. | 84.59°C | b. | 24.63°C | d. | 92.64°C | | | | |
|
|
|
27.
|
How
many moles of helium gas are contained in a 4.0-L flask at STP? a. | 0.045
mol | c. | 0.17
mol | b. | 0.089
mol | d. | 89
mol | | | | |
|
|
|
28.
|
A
steel tank with a volume of 9.583 L contains N2 gas under a pressure of 4.972 atm at 31.8
°C. Calculate the number of moles of N2 in the tank. a. | 0.002
mol | c. | 0.525
mol | b. | 0.018
mol | d. | 1.90
mol | | | | |
|
|
|
29.
|
According to Gay-Lussac’s law: a. | pressure is
inversely proportional to volume at constant temperature. | b. | pressure is
directly proportional to temperature at constant volume. | c. | volume is
inversely proportional to temperature at constant pressure. | d. | volume is
directly proportional to temperature at constant pressure. | | |
|
|
|
30.
|
What
volume of oxygen is needed to react with solid sulfur to form 6.20 L of sulfur
dioxide? a. | 6.20
L | c. | 12.4
L | b. | 7.20
L | d. | 99.2
L | | | | |
|
|
|
31.
|
How
many moles of steam are produced at 2.00 atm and 202.0°C by the complete combustion of 12.50 L
of methane gas? a. | 0.001
mol | c. | 0.640
mol | b. | 0.012
mol | d. | 1.28
mol | | | | |
|
|
|
32.
|
When
a milkshake is taken in through a straw at a pressure of 0.071 atm, the straw contains 5.0 mL of
liquid. How much liquid is consumed at 0.092 atm? a. | 0.10 mL | c. | 6.3 mL | b. | 3.9
mL | d. | 7.8
mL | | | | |
|
|
|
33.
|
In a
hospital, oxygen is administered to patients at 3.0 atm in a hyperbaric oxygen chamber. Oxygen gas,
measuring 600.0 L, is compressed in a cylinder at 160.0 atm. What volume of oxygen can a cylinder
supply at the given pressure? a. | 11 L | c. | 11 ´ 103 L | b. | 32
L | d. | 32 ´ 103
L | | | | |
|
|
|
34.
|
A
2.50 L balloon is filled with water at 2.27 atm. If the balloon is squeezed into a 0.80 L beaker and
does NOT burst, what is the pressure of water in the balloon? a. | 0.72
atm | c. | 7.1
atm | b. | 0.88
atm | d. | 8.8
atm | | | | |
|
|
|
35.
|
A
balloon is filled with 3.50 L of water at 24.0°C and 2.27 atm. If the balloon is placed outdoors
on a hot day at a temperature of 34.0°C, what is the volume of the balloon at constant
pressure? a. | 2.47
L | c. | 3.61
L | b. | 3.38
L | d. | 8.19
L | | | | |
|
|
|
36.
|
The
volume of a sample of helium is 4.50 mL at 20.0°C and 203.0 kPa. What will its volume be at
10.0°C and 203.0 kPa? a. | 2.25 mL | c. | 4.34 mL | b. | 3.78
mL | d. | 6.85
mL | | | | |
|
|
|
37.
|
The
volume of a 24.0-g sample of methane gas is 22.8 L at 40.0°C and 4.00 atm. What will its volume
be at 68.0°C and 4.00 atm? a. | 20.9 L | c. | 38.7 L | b. | 24.8
L | d. | 40.8
L | | | | |
|
|
|
38.
|
A
40.0-L sample of fluorine is heated from 90.0°C to 186.0°C. What volume will the sample
occupy at the higher temperature? a. | 19.3 L | c. | 50.5 L | b. | 31.6
L | d. | 82.6
L | | | | |
|
|
|
39.
|
A
welding torch requires 3200 L of ethylene gas at 3.00 atm. What will be the pressure of the gas if
ethylene is supplied by a 250.0-L tank? a. | 0.231 atm | c. | 38.4 atm | b. | 2.34
atm | d. | 45.4
atm | | | | |
|
|
|
40.
|
The
volume of a gas is 1.50 L at 30.0°C and 1.00 atm. What volume will the gas occupy if the
temperature is raised to 134.0°C at constant pressure? a. | 0.331
L | c. | 2.01
L | b. | 1.11
L | d. | 6.70
L | | | | |
|
|
|
41.
|
A
sample of carbon dioxide occupies a 2.54 dm3 container at STP. What is the volume of the
gas at a pressure of 150.0 kPa and a temperature of 26.0°C? a. | 0.180
dm3 | c. | 2.24
dm3 | b. | 1.88 dm3 | d. | 4.65 dm3 | | | | |
|
|
|
42.
|
One
liter of a gas has a pressure of 150.00 kPa at 25.0°C. If the pressure increases to 600.0 kPa
and the temperature to 100.0°C, what would be the new volume of the gas? a. | 0.200
L | c. | 1.00
L | b. | 0.312
L | d. | 2.90
L | | | | |
|
Matching
|
|
|
Match the
terms below with their correct definitions. a. | Avogadro’s principle | e. | Gay-Lussac’s law | b. | Boyle’s
law | f. | ideal gas
constant | c. | Charles’s law | g. | ideal gas law | d. | combined gas
law | h. | molar
volume | | | | |
|
|
|
43.
|
Equal
volumes of gases at the same temperature and pressure contain equal numbers of
particles.
|
|
|
44.
|
One mole of
any gas will occupy a volume of 22.4 L at STP.
|
|
|
45.
|
R represents
the relationship among pressure, volume, temperature, and number of moles of gas
present.
|
|
|
46.
|
Temperature,
pressure, and volume are related for a fixed amount of gas.
|
|
|
47.
|
The physical
behavior of an ideal gas can be expressed in terms of the pressure, volume, temperature, and number
of moles of gas present.
|
|
|
48.
|
The pressure
of a given mass of gas varies directly with the kelvin temperature when the volume remains
constant.
|
|
|
49.
|
The volume
of a given amount of gas held at a constant temperature varies inversely with the
pressure.
|
|
|
50.
|
The volume
of a given mass of gas is directly proportional to its kelvin temperature at constant
pressure.
|